Safety and Toxicity Data of Theracurmin®
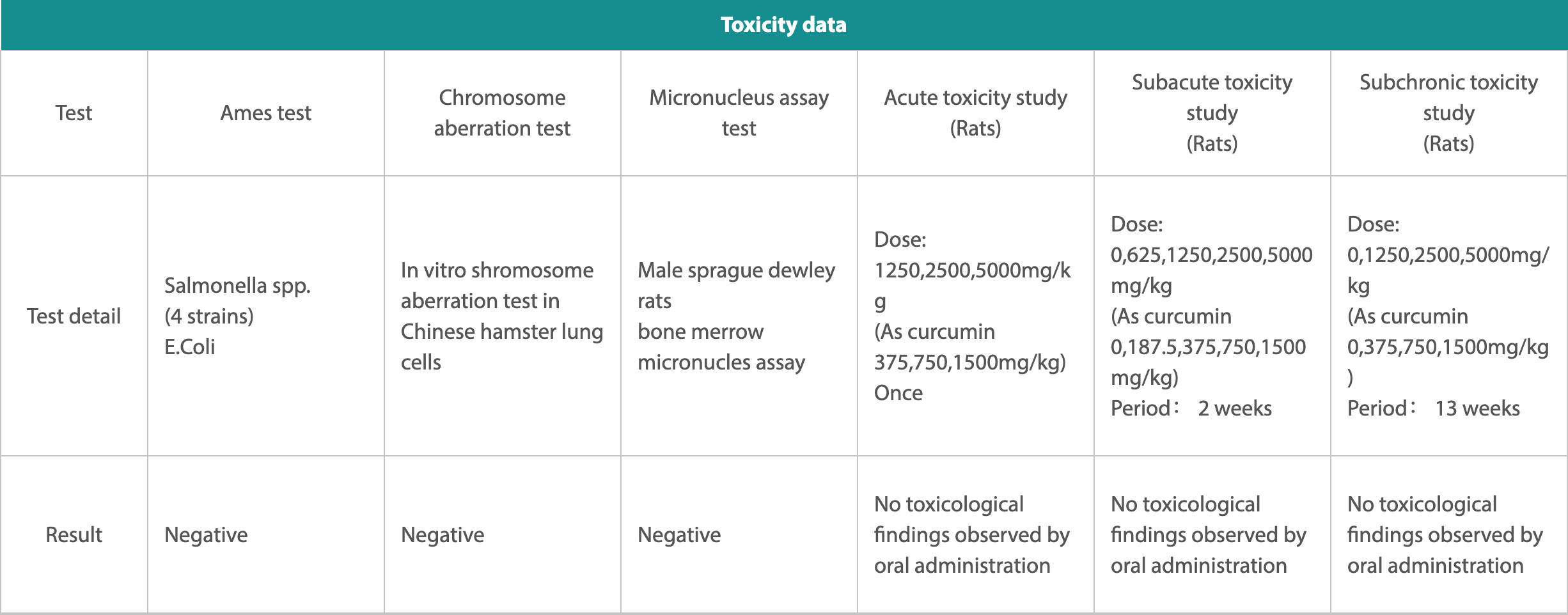
The toxicity assessment and safety test results of our well-developed Theracurmin® are as follows.
1. Ames test had been performed with the Salmonella spp. (4 strains) E. Coli and test shows result as negative.
2. Chromosome aberration tests have been performed in vitro chromosome aberration test in Chinese hamster lung cells and tests show results as negative.
3. Micronucleus assay tests have been performed with Male Sprague dewley rats bone marrow micronucles assay and again tests show results as negative.
4. Acute toxicity studies (Rats) performed with a dose of 1250,2500,5000mg/kg as curcumin 375,750,1500mg/kg once resulted in no toxicological findings observed by oral administration.
5. Subacute toxicity study (Rats) test performed with a dose of 0,625,1250,2500,5000mg/kg as curcumin 0,187.5,375,750,1500mg/kg with a period of 2 weeks resulted in no toxicological findings observed by oral administration.
6. Subchronic toxicity study (Rats) test performed with a dose of 0,1250,2500,5000mg/kg as curcumin 0,375,750,1500mg/kg with a period of 13 weeks resulted in no toxicological findings observed by oral administration.